|
Tip Archives - 2004 | Back to Archive Index
December 2004- MODIFIERS: -25 & -59 What are the Differences?
Modifiers: Differences Between the -25 and -59
It is sometimes difficult to determine which modifier is correct for your particular situation. Shown below are the uses of the-25 and -59 modifiers, and the situations where they would be properly applied.
Modifiers Definitions
The -59 modifier should be used to identify procedures/services that are not normally reported together, but are appropriate under the circumstances. This may represent a different procedure or surgery, different site or organ system, separate lesion or separate injury not normally encountered or performed by the same physician.
Use the -25 modifier to identify a significant, separately identifiable Evaluation and Management (E/M) service by the same physician on the date a procedure is performed.
Correct Application of Modifiers
Use the modifiers to report services that fall within the National Correct Coding Initiative, which are "distinct procedural services". One use of modifier -59 would be with the 2005 CPT PET and PET/CT (78811-78816) codes with a medically necessary diagnostic CT performed and reported on the same day with the PET or PET/CT scan. More information about the National Correct Coding Initiative can be found at www.cms.hhs.gov/medlearn/ncci.asp.
The -25 modifier should be applied to E/M services that are "significant or separately identifiable" from the procedure that have the same-day or a 10-day global period. This modifier can be used along with the -59 modifier when you have an E/M service that falls within the global package and the National Correct Coding Initiative. However, the -59 modifier is not a replacement for the global modifier -25.
November 2004- HCPCS Codes Effective January 1, 2005 & New Process for 2006 Centers for Medicare and Medicaid Services (CMS)
During a CMS open door forum on October 27, 2004, Cindy Hake, Chairperson of the HCPCS National Panel stated "the 2005 HCPCS file should be posted on the CMS web site soon for codes effective January 1, 2005. " At the time of the posting of this tip the file was not yet available, so please check the following CMS URLs during the next few weeks to obtain this important coding information. Facilities must implement these new and revised codes by January 1, 2005 into your charge description masters.
Alpha-Numeric HCPCS Files:
http://www.cms.hhs.gov/providers/pufdownload/default.asp#alphanu
http://www.cms.hhs.gov/providers/pufdownload/anhcpcdl.asp
Background & History of HCPCS
The Healthcare Common Procedure Coding System (HCPCS) was established in 1978 to provide a standardized coding system for describing the specific items and services provided in the delivery of health care. Such coding is necessary for Medicare, Medicaid, and other health insurance programs to ensure that insurance claims are processed in an orderly and consistent manner. Initially, use of the codes was voluntary, but with the implementation of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) use of the HCPCS for transactions involving health care information became mandatory.
The HCPCS is divided into two principal subsystems, referred to as Level I and Level II. Level I is comprised of the CPT-4, a numeric coding system maintained by the American Medical Association (AMA) to identify medical services and procedures furnished by physicians and other health care professionals. The Level II HCPCS is a standardized coding system that is used primarily to identify products, supplies, and services not included in the CPT-4 codes. It is maintained and distributed by CMS, in conjunction with private payer organizations.
During 2005 and effective 2006, CMS will significantly change the HCPCS application process to allow for a more open and public process. The most significant change being the ability of anyone to see the proposed changes on the CMS web site and have an opportunity to supply comments to the HCPCS panel regarding the application.
The major new changes to the 2006 HCPCS coding process will include:
• Expansion of the Public Meetings- The current public meetings related to durable medical equipment (DME) will be expanded to include all public requests for HCPCS products, supplies and services. Agenda items for the meetings will be published in advance, including descriptions of the coding requests, the requestor, and the name of the product or service. This change will provide more opportunities for the public to become aware of coding changes under consideration, as well as opportunities for public input into decision-making.
• Appeals Process- CMS expects to implement an appeals process in the 2007 coding cycle, whereby denied applicants will be allowed to appeal the decision, and given an opportunity to have their application reconsidered during the same coding cycle.
• Public Notice of Decisions- All preliminary decisions will be published on the CMS web site prior to public meetings to facilitate effective public discussion and comment. Final decisions based on public comments, as well as decisions resulting from appeals, will be similarly published.
• Revision of the HCPCS Code Application Form
• Reduction in Marketing Data for Non-drug items
• Consideration of national Medicaid program operating needs
CMS provides a HCPCS Application Process (2005-2006) chart at:
http://www.cms.hhs.gov/medicare/hcpcs/hcpcs_process.pdf
The revised HCPCS coding process will be phased in over the next 18 months. The first change in the process is an earlier application deadline of January 3, 2005. This change will permit expanded opportunities for public comment on preliminary coding decisions compared to the current coding update schedule.
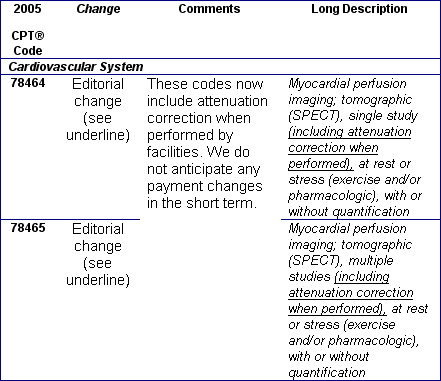
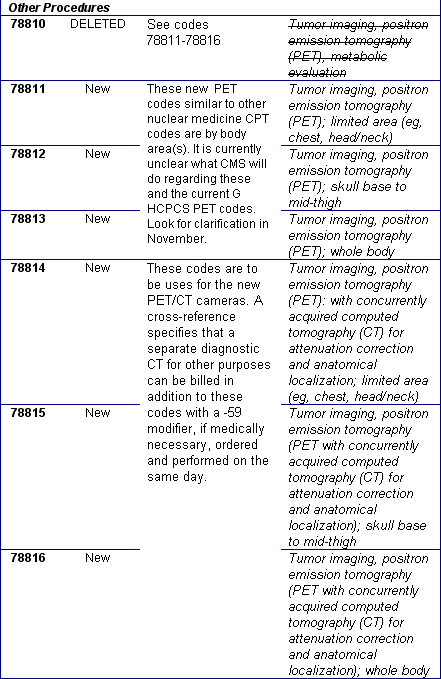
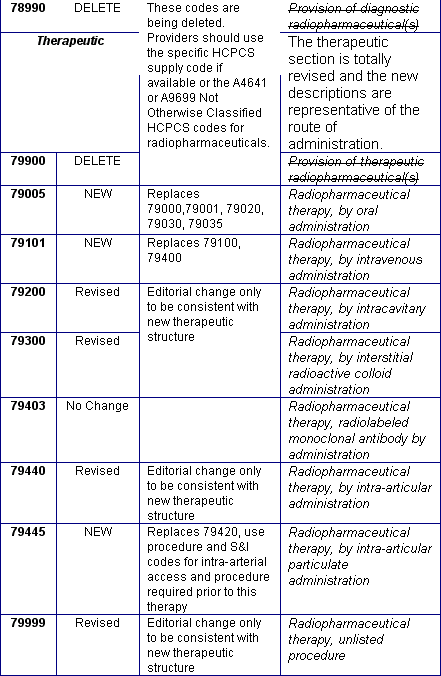
October 2004: New/Revised and Deleted Nuclear Medicine CPT® Codes Effective January 1, 2005
You will no doubt receive your 2005 CPT® books soon. The table below is a preview of the nuclear medicine changes. These codes need to be implemented or deleted from your charge master by January 1, 2005. The 90 day grace period has been eliminated. Regarding the current G series HCPCS codes for PET tumor imaging: we have to wait until November to see what Medicare will do regarding these new PET CPT codes. Many of the professional societies have requested CMS to drop the numerous G HCPCS PET codes and adopt the CPT PET and PET/CT codes. We anticipate that many private payers will choose to use the new PET CPT codes. We recommend that you begin conversations with your top private payers to prepare your charge masters properly for the January 1, 2005 implementation and stay tuned for CMS clarification in November.
New/Revised and Deleted 2005 CPT® Codes Nuclear Medicine Section,
January 1, 2005
September 2004: New Hospital Revenue Codes for Radiopharmaceuticals Go Into Effect on October 1, 2004
Are You Ready?
The HealthInsurance Portability and Accountability Act (HIPAA) Transaction and Code Set Rule requires providers to use the medical code set that is valid at the time the service is rendered. This important coding information applies to ALL coding systems.
Many articles and communications have discussed this rules implementation as it applies to HCPCS, CPT and ICD-9 CM codes. But hospitals should pay equal attention to the National Uniform Billing Committee (NUBC) revenue code changes, which are implemented less frequently. According to one NUBC committee member, ³the HIPAA requirement regarding current codes applies to the hospital revenue codes.² Will you be ready for the nuclear medicine revenue code changes?
NEW and REVISED Hospital Nuclear Medicine Revenue Codes
Before October 1, 2004, hospitals have had several choices of revenue codes to report along with the radiopharmaceutical HCPCS codes. CMS states that hospitals should continue to use revenue code 0636 when reporting radiopharmaceuticals or drugs eligible for separate continued payment, status indicator K. Concerning radiopharmaceuticals with a packaged status indicator N, in its April 2, 2003 transmittal, CMS directed hospitals to use revenue codes 025X or 062X. Revenue code 025X is "Pharmacy" and 0255 is "Drugs Incident to Radiology." Code 0621 is described as "Supplies Incident to Radiology" and code 0622 as "Supplies Incident to Other Diagnostic Services."
Given the codes and descriptions currently available, the Society of Nuclear Medicine (SNM) advises hospitals to choose revenue code 0622 for Nuclear Medicine Drugs and Radiopharmaceuticals that are packaged and designated as status indicator N prior to October 1, 2004.
Starting October 1, 2004, two new radiopharmaceutical revenue codes will be available for hospitals. Charge description masters should use revenue code 0343 for all diagnostic radiopharmaceuticals and code 0344 for all therapeutic radiopharmaceuticals, regardless of status indicator K or N.
For all status K radiopharmaceuticals, hospitals must continue to report the HCPCS code along with the new revenue code. We recommend that hospitals also report the specific HCPCS codes for status N radiopharmaceuticals, but they are not required to do so.
The new and revised nuclear medicine revenue codes, effective October 1, 2004 are referenced in CMS transmittal 81 published on February 6, 2004, under the title ³NUBC UB-92 Medicare Claims Processing.² (Transmittal 81 is available at http://www.cms.hhs.gov/manuals/pm_trans/R81CP.pdf)
This is a long transmittal, and to assist you we have extracted the items that pertain to the nuclear medicine section and radiopharmaceuticals.
New Radiopharmaceutical Revenue Codes
CMS Transmittal 81, published on February 6, 2004, instructs Medicare Hospital Contractors to implement additions and revisions to the nuclear medicine revenue codes as of October 1, 2004. Hospitals should prepare to add these radiopharmaceuticals revenue codes to their charge description masters, remembering that they DO NOT become effective until October 1, 2004.
The tables below show the new and revised nuclear medicine revenue codes. CMS and SNM educational materials will be provided in future issues and on the SNM web site.
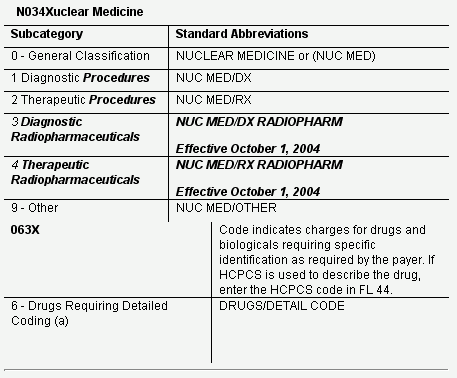
NOTE: (a), Effective October 1, 2004, charges for drugs and biologicals requiring specific identifications as required by the payer (except for radiopharmaceuticals, which are reported under Revenue Codes 0343 and 0344). If HCPCS are used to describe the drug, enter the HCPCS code in Form Locator 44. The specified units of service to be reported are to be in hundreds (100s), rounded to the nearest hundred (no decimal).
August 2004: Medicare 2005 Proposed Physician Fee Schedule Summary
On July 27th, 2004 the Centers for Medicare and Medicaid Services (CMS) posted on their web site the 2005 Proposed Physician Fee Schedule. The proposed rule will be published in the August 5, 2004 Federal Register. Comments will be accepted until September 24, 2004. CMS plans to publish the final rule by November 1st 2004, with an effective date of January 1, 2005.
Not a surprise is the proposed conversion factor (CF) $37.8975, current CF $37.3374, which is a payment increase of 1.5 percent, as mandated in the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA). The implementation of the MAA essentially avoided the projected update of minus 3.7 percent for payment rates for physicians in 2005.
There are a host of new preventative benefits, which were authorized by the MMA 2003. Among those new proposed services is “Welcome of Medicare Physical” an initial preventative examination for all new Medicare beneficiaries. Additionally, this proposed rule provides of cardiovascular screening blood tests for the detection of cardiovascular disease or related abnormalities and coverage for diabetes screening tests. The new CMS administrator, McClellan stated, “We intend to use these new benefits to re-direct Medicare to become a truly prevention-focused program.”
Drug & Administrations
* Adjusts payments for Part B-covered drugs and the administration services o CMS has left the door open to consider further changes to coding and payment for administering drugs based on a review currently underway by the American Medical Association (AMA) Current Procedural Terminology (CPT) Editorial Panel. This outcome will effect payments for the injection of cold antibodies given prior to “hot” radioactive treatments such as with Zevalin® and Bexxar®.
* The standard payment rate for most Part B drugs will be set at 106 percent of the average sales price (ASP), as reported by the manufacturers. Preliminary data on ASP for 32 widely used drugs are published o The proposed rule also identifies an alternate method for payment when ASP is excessive. “The Secretary may disregard the average sales price for a drug or biological that exceeds the widely available market price for the average manufacturer price for such drug or biological by the applicable threshold percentage.”
Other Items to Note
* There is a proposal to establish a separately billable supplying fee, payment rate of $10.00 for each prescription, effective January 1, 2005. This supplying fee would be allowable for immunosuppressive drugs, oral anticancer chemotherapeutic drugs, and oral anti-emetic drugs as part of an anticancer chemotherapeutic regimen.
* Medicare also proposes to cover the routine costs of clinical trails involving Category A devices
Radiology
* CMS proposes to remove restrictions on payment for higher-cost low osmolar contrast materials (LOCM) Payment for LOCM will be ASP plus six percent and taking into account the minus 8% for those costs included in the practice expense. See A4644 through A4646 in the CMS tables.
* The MMA excluded payment for screening and diagnostic mammograms from the OPPS. CMS proposes to set payments for both screening and diagnostic mammograms under the physician fee schedule. More details will be in the HOPPS rule available in early August.
* Discussions regarding Set-up of Portable X-ray Equipment Codes o Q0092 Set up of portable x-ray equipment RVU, 0.33, national payment $12.32 o R0070 & R0075 Portable x-ray transportation (carrier priced $89-$112.) o CMS is interested in pubic comments on whether they should pursue national pricing for portable x-ray set-up outside of the non physician work pool or local carrier pricing for 2005 or whether they should continue to price the service in the non physician work pool.
Nuclear Medicine
* Parathyroid Imaging, CPT 78070 based on comments sent by SNM, ACR and the AMA RUC, this procedure involves multiple imaging sessions. CMS agrees to crosswalk the charge-based RVUs from CPT 78306, whole body imaging to this procedure (see table below)
* Increased payment by 7% for J9310 (RITUXAN) $438.38
* Increased payment by 5% for J 0152 Adenosine $69.78
Extracted From Table B 2004 and proposed 2005 MPFS for 78070
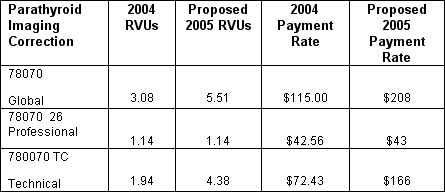
From Practice Expense Section
* CMS is proposing to delete the current “room” designation for the radiopharmaceutical receiving area and, in its place, list separately the equipment necessary for each procedure as individual line items because there does not appear to be a standard configuration for such a room across the nuclear medicine codes.
Cardiology
* J 0152 Adenosine increased payment by 5% to $69.78
* Limitation on coverage of cardiovascular screening tests. Payment may be made for cardiovascular screening tests performed for an asymptomatic individual only if the individual has not had the screening tests paid for by Medicare during the preceding 59 months following the month in which the last cardiovascular screening tests were performed.
July 2004: Hospital Brand vs. Generic Radiopharmaceuticals Centers for Medicare and Medicaid Services (CMS)
In April 2004, CMS implemented Transmittal 112, which describes changes to payments for brand name vs. generic drugs, biologicals, and radiopharmaceuticals under the Hospital Outpatient Prospective Payment System. CMS states that the new codes ³are required to enable us to differentiate between the payment amount required under the MMA (Medicare Prescription Drug, Improvement and Modernization Act of 2003) for a brand name drug and the payment amount required under the MMA for its generic form."
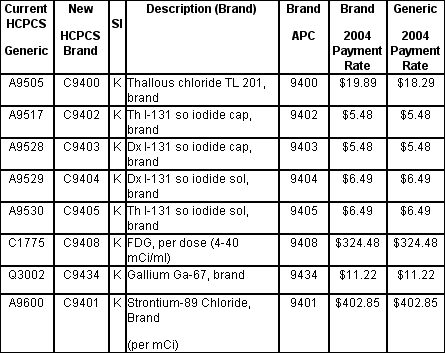
These new HCPCS codes have caused much confusion for nuclear medicine departments trying to properly identify, track, document, and code the (now) eight branded radiopharmaceuticals.
Hospitals employing the new HCPCS codes face a wide range of identification, documentation, and charge description master implementation issues. In both Transmittal 112 and Transmittal 194, which announce and list the new codes, CMS does not identify which manufactures were considered the "branded" radiopharmaceuticals. CMS officials plan to leave this task for hospitals and manufacturers to work out.
Many hospitals purchase from nuclear pharmacy distributors, who in turn buy radiopharmaceuticals from multiple manufacturers. Nuclear pharmacy invoicing and tracking may not clearly identify to hospitals the manufacturer of a dose to be administered to a patient. Further, hospitals may not pay a different price based on manufacturer. Prices are negotiated in advance and take into consideration all the variables for timely delivery of a radiopharmaceutical to a facility.
Of the eight new branded radiopharmaceutical HCPCS codes, only one includes a payment rate different from that of its generic counterpart (see attached table). Additionally, these new C series HCPCS codes can only be used in the hospital outpatient Medicare program. As far as we are aware, private payers do not recognize C series codes.
Given the administrative burden, and the minimally- or non-increased rates, we caution hospitals in regard to the proper use of these new "branded" radiopharmaceutical codes. For clarification, if hospitals are unclear if they are administering a ³branded² radiopharmaceutical they may choose continue to code and bill using the generic version of the same code. For those hospitals choosing to implement the codes we recommend careful review and strict documentation on a patient by patient basis.
June 2004: Local Coverage Determination (LCD) Replaces LMRPs Centers for Medicare and Medicaid Services (CMS)
Effective December 7, 2003, the Centers for Medicare and Medicaid Services (CMS) have started using local coverage decisions (LCDs) rather than local medical review policies (LMRPs). Although this may appear to be just a name change, there are differences between the LMRPs and the new LCDs.
Specifically, the new LCDs focus on “reasonable and necessary” information, while the old LMRPs included benefit category and statutory exclusion provisions. LMRPs also contained a host of other coding information not directly related to medical necessity. In addition, CMS has given instructions to contractors that LCDs should not address fraud or fraudulent activities, and should only refer to issues that are “not reasonable and necessary.”
The final rule establishing LCDs was published on November 11, 2003, and became effective on December 7, 2003. Medicare contractors began using LCDs as of this date, and will transition all LMRPs to LCDs over the next two years.
May 2004: CMS Corrections for Nuclear Medicine
Keeping current with updates is a challenge for all of us, however it is necessary. The Centers for Medicare and Medicaid Services (CMS) provide Transmittals and Change Requests outside the traditional rule-making and implementation process. In the Reimbursement Links Web site, we provide easy access to the CMS manuals and transmittals Web sites. We encourage you to review these documents for information that is important to your facility. Additionally, professional societies are often notified before new information is published. Therefore, frequent visits to these Web sites is recommended, in addition to visiting the CMS Web sites.
Hospital Outpatient Prospective Payment System (HOPPS) Corrections
Quarterly Update of Addendum A & B
Beginning April 1, 2004 CMS will post a revised Addendum A and B on the hospital Outpatient Prospective Payment System (OPPS) Web site that reflects quarterly changes and corrections in OPPS. This updated file will also contain codes that are deleted due to the grace period that ends on April 1, 2004. Those codes will be noted as “DG” in the column labeled “cond”.; This month, we spotlight several CMS corrections for nuclear medicine that have occurred since January 1, 2004 and have been implemented in the April to July time period as follows:
CMS Transmittal 113, published on February 27, 2004 and implemented on April 5, 2004, notes nine radiopharmaceutical payment corrections as listed in the Table below:
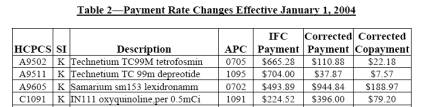
The corrected payment rates in the above table are in effect for services furnished on or after January 1, 2004. CMS instructed the Fiscal intermediaries to adjust the payments for claims that were incorrectly paid for services that were furnished from January 1, 2004 through March 31, 2004.
CMS Transmittal 132, published on March 30, 2004 and implemented on April 5, 2004, notes that Ammonia N-13 per dose HCPCS A9526 and Q4078 were incorrectly assigned status indicator “N” in the December 31, 2003 Correction Notice (68FR75442). Per this transmittal, effective January 1, 2004, the correct status indicator for these HCPCS codes is “K”. Published payment rate for these codes is listed as $162.63. However, the Q4078 code is now deleted, so be sure to update your charge description masters with the current HCPCS code A9526 Ammonia per dose.
Physician Fee Schedule Correction and CCI Edit Correction for 78804
Pending CMS Publications
The Society of Nuclear Medicine is currently reporting on their web site an article which states, CMS has notified the SNM of two corrections which will be published soon regarding the new CPT code 78804 Radiopharmaceutical localization of tumor or distribution of radiopharmaceutical agent(s); whole body, requiring two or more days imaging. The corrections are in response to letters written by the professional society and details of these letters and CMS response as well as the pending corrections can be located on the SNM website at www.snm.org.
April 2004: CMS Open Door Forums
The Centers for Medicare and Medicaid Services (CMS) providers an excellent opportunity through their Open Door Forums for you to meet and discuss your issues with CMS staff directly. CMS provides an 800 telephone number for you and your staff to dial in and listen as well as ask questions of top CMS officials. The staff begins each program with updates followed by opening up the telephone lines for provider questions. If answers can not be provided immediately each caller is given a person to follow up with.
Signing up is easy, just go to the CMS web site and click on Open Door Forms and then click on Open Door Registration, see link below. You will be provided with a list as below and all you need do is select the topics you are interested in and give them your e-mail address. A notification and dial in number will be provided via e-mail.
http://www.cms.hhs.gov/opendoor/listservs.asp
Ambulance Open Door Forum
Disability Open Door Forum
Diversity Open Door Forum
End-Stage Renal Disease & Clinical Laboratories Open Door Forum
Health Plans Open Door Forum
Home Health, Hospice & Durable Medical Equipment Open Door Forum
Hospital Open Door Forum
Low Income Health Access Open Door Forum
New Freedom Initiative Open Door Forum
Nurses & Allied Health Professionals Open Door Forum
Pharmaceutical, Pharmacy, and Device Manufacturers Open Door Forum
Physician Open Door Forum
Rural Health Open Door Forum
Skilled Nursing Facility/Long Term Care Open Door Forum
Remember that these programs are for your use and a way for CMS to provide you with the answers to your questions, specifically and immediately, so sign up and participate today.
March 2004: Medicare Prescription Drug, Improvement and Modernization Act (MMA) of 2003 provide changes to the Single Drug Pricer (SDP)
The Centers for Medicare and Medicaid Services (CMS) notified all Medicare providers who bill either carriers or fiscal intermediaries for drugs under Medicare Part B regarding a new basis for payment mandated with the enactment of the MMA.
As of January 1, 2004 based on the MMA, drugs and biologicals will be paid at 85% of the Average Wholesale Price (AWP) for the Healthcare Common Procedure Coding System (HCPCS) payment amount based on the April 1, 2003 Single Drug Pricer (SDP). Additionally, the Single Drug Pricer mailbox at SDP@cms.hhs.gov will no longer be available. Questions must be sent to the appropriate CMS regional office which can be located on the CMS Web site or under this site links to reimbursement resources web page.
Effective January 30, 2004 CMS alerted providers that CMS’ Web site issued corrected drug and biological pricing files. Be sure to update your files with the January 30th information located at http://cms.hhs.gov/providers/drugs/default.asp (MMA drug Payment limits Pricing Files For Dates of Services 1/1/2004 and After – Revised).
Affected providers should note that payers have been instructed to apply these changes to new claims received and they are not automatically adjusting claims previously paid. These Medicare contractors have been instructed to adjust claims that are brought to their attention by the provider. Therefore you can submit an adjustment to your Medicare contractor and it will be processed.
Providers should continue to look for updates on a quarterly basis via files published on the CMS Web site. We anticipate updated files effective April 1, July 1 and October 1, 2004.
February 2004: Advanced Beneficiary Notice ABNs
Are you utilizing Advanced Beneficiary Notices (ABN) to inform your Medicare patients that procedures may be medically unnecessary?
Centers for Medicare and Medicaid Services (CMS)
The provider is responsible for knowing the Medicare policies for their state and for informing a patient in writing when Medicare is likely to deny payment for a planned procedure. Failing to inform a patient that a procedure is considered not medically necessary, and failing to bill the patient for such procedures, could cause a provider to be held liable under the provisions of Limitation of Liability laws (Title XVIII, section 1879).
The way to avoid problems is to issue an Advance Beneficiary Notice (ABN). An ABN informs a patient that a particular procedure, even though it was ordered by his/her physician, may not be considered medically necessary by Medicare, and that if payment is denied by Medicare, the patient will be responsible for paying for the procedure.
A service might require an ABN when the primary diagnosis (ICD-9) code does not qualify as “medically necessary and reasonable” for the patient’s specific condition. Or a procedure may have restrictions on how frequently a procedure may be repeated. A provider needs to stay current regarding all published local and national coverage decisions which contain this valuable information. Providers can obtain National Coverage Determinations (NCD) and Local Medical Review Policies (LMRPs) for their Medicare Carrier or Fiscal Intermediary from local or national web sites (go to Links to Reimbursement Resources and then either Intermediary/Carrier Directory or Medicare Coverage Database).
Complete ABN instructions have been published on the CMS Web site at http://cms.hhs.gov/medicare/bni/AB02168.pdf.
January 2004: 2004 Nuclear Medicine CPT® and HCPCS codes
Did you know that there are new and modified Nuclear Medicine CPT® Codes effective January 1, 2004? The new and/or revised Nuclear Medicine CPT® codes are:
78800 Radiopharmaceutical localization of tumor or distribution of radiopharmaceutical agent(s); limited (For specific organ, see appropriate heading)
78802 Radiopharmaceutical localization of tumor or distribution of radiopharmaceutical agent(s); whole body, single day imaging
78804 Radiopharmaceutical localization of tumor or distribution of radiopharmaceutical agent(s); whole body, requiring two or more days imaging
79100 Radiopharmaceutical therapy, polycythemia vera, chronic leukemia, each treatment by intravenous injection (For monoclonal antibody therapy use 79403)
79400 Radiopharmaceutical therapy, nonthyroid, non hematologic by intravenous injection (Do not report 79400 in conjunction with 79403) (For monoclonal antibody therapy, use 79403)
79403 Radiopharmaceutical therapy, radiolabeled monoclonal antibody by intravenous infusion (For pre-treatment imaging, see 78802, 78804) (Do not report 79403 in conjunction with 79400) Additionally, a cross-reference was added following code 77750 to direct the user to report code 79403 for administration of radiolabeled monoclonal antibodies.
Some new and/or revised Nuclear Medicine Healthcare Common Procedure Coding System HCPCS codes effective January 1, 2004 are:
A9517 Supply of Radiopharmaceutical Therapeutic Imaging Agent, I-131 Sodium Iodide Capsule, per mCi – (Short Description: Th I-131 so iodide cap millic)
A9526 Supply of Radiopharmaceutical Diagnostic Imaging Agent, Ammonia N-13, per dose
A9528 Supply of Radiopharmaceutical Diagnostic Agent, I-131 Sodium Iodide Capsule, per millicurie
A9529 Supply of Radiopharmaceutical Diagnostic Agent, I-131 Sodium Iodide Solution, per millicurie
A9530 Supply of Radiopharmaceutical Therapeutic Agent, I-131 Sodium Iodide Solution, per millicurie
A9531 Supply of Radiopharmaceutical Diagnostic Agent, I-131 Sodium Iodide, per microcurie (up to 100 microcuries)
A9532 Supply of Radiopharmaceutical Therapeutic Agent, Iodinated I-125 Serum Albumin, 5 microcuries
A9533 Supply of Radiopharmaceutical Diagnostic Imaging Agent, I-131 Tositumomab, per millicurie NOTE: Except in Hospital Outpatient Setting use C1080 see below.
A9534 Supply of Radiopharmaceutical Therapeutic Imaging Agent, I-131 Tositumomab, per millicurie NOTE: Except in Hospital Outpatient Setting use C1081 see below.
NOTE: C codes below are used primarily in the Medicare Hospital Outpatient Setting unless specified by a payer.
- C1080 Supply of Radiopharmaceutical Diagnostic Imaging Agent, I 131 Tositumomab per dose
- C1081 Supply of Radiopharmaceutical Therapeutic Imaging Agent, I 131 Tositumomab per dose
- C1082 Supply of Radiopharmaceutical Diagnostic Imaging Agent, Indium-111 Ibritumomab Tiuxetan per dose
- C1083 Supply of Radiopharmaceutical Therapeutic Imaging Agent, Yttrium 90 Ibritumomab Tiuxetan per dose
J0152 Injection, Adenosine, 30 mg (Not to be used to report any adenosine phosphate compounds; instead use A9270)
Note: Most HCPCS codes have a three-month grace period when either the old or new code may be used and receive payment during this grace period. Always check with your local payer regarding proper selection of codes.
A complete list of the 2004 HCPCS codes can be located at: http://www.cms.hhs.gov/providers/pufdownload/anhcpcdl.asp. Also available are some coding educational materials showing the proper use of these and other nuclear medicine codes available on the SNM Web site at: www.snm.org.
2004 Medicare payment rates for these and other codes in the Ambulatory Payment Classification (APC) and Physician Fee Schedule (PFS) are now available on government web sites, see below URL links for complete information.
Medicare APCs:
http://www.cms.hhs.gov/regulations/hopps/2004ifc/
http://a257.g.akamaitech.net/7/257/2422/14mar20010800 /edocket.access.gpo.gov/2004/pdf/03-32322.pdf
Medicare PFS:
http://www.cms.hhs.gov/regulations/pfs/2004fc/1372fc/1372fc.asp
Or check your local Medicare Carrier Web site for specific Local Payment Rates.
|