Effective January 1, 2014, the Centers for Medicare and Medicaid Services (CMS) made it mandatory to report a clinical trial number when submitting claims for items and services provided in a clinical trial that are qualified for coverage as specified in the National Coverage Determination (NCD) Manual, Publication 100-03, Section 310.1. Previously, the clinical trial number was reported on a voluntary basis.
When a new study is listed in the National Library of Medicine (NLM) Clinical Trials data base, a clinical trial number is assigned by the NLM Clinical Trials.gov website. This number is prominently displayed on each specific study’s page and is preceded by the letters “NCT.” This number is used by CMS to identify all items and services provided to a beneficiary during their participation in a clinical trial, clinical study, or registry including studies covered under coverage with evidence development (CED), the Medicare Clinical Trial Policy, or a CMS-approved investigational device exemption (IDE).
The use of this number helps CMS to track Medicare payments, ensure research information is used to inform coverage decisions, and to verify that the study targets issues that are relevant to the Medicare population. Please check CMS’ clinical trials registry website: http://www.cms.gov/Medicare/Medicare-General-Information/MedicareApprovedFacilitie/index.html to verify the validity of a clinical trial, study or registry. Also, you can search for studies and the associated NCT Identifier on the National Library of Medicine (NLM) website: http://clinicaltrials.gov/
The Clinical Trails.gov Identifier associated with National Oncologic PET Registry (NOPR) is NCT00868582 and can be verified at:
https://clinicaltrials.gov/ct2/show/NCT00868582?term=nopr&rank=1
Please note the above National Clinical Trials identifier is the same NCT identifier that was used to report both F-18 FDG-PET and NaF-18 studies. The FDG-PET registry was closed effective June 11, 2013 when CMS issued a final decision memorandum which called for the end of the prospective data collection requirements under Coverage with Evidence Development (CED) for all oncologic indications for FDG-PET. This decision applied only to the FDG-PET registry; the NaF-PET registry remains open. Claims reporting items and services for NaF-PET studies must continue to report NCT00868582 on the claim. See below for details.
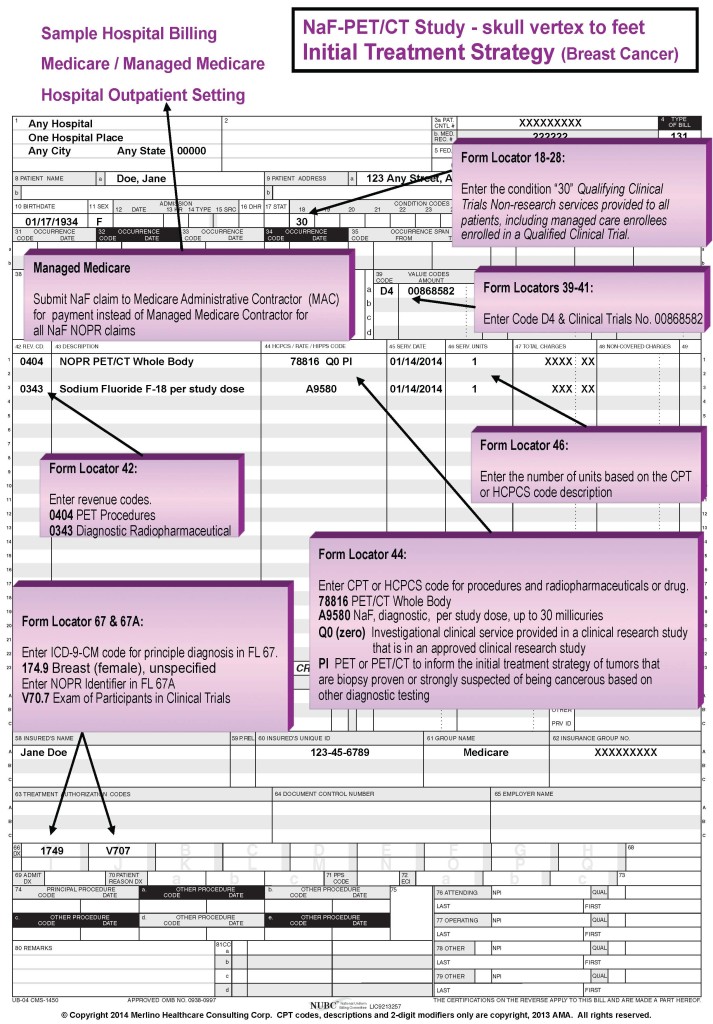
For facility/institutional claims, submitted on Form UB-04 (CMS 1450), enter value code “D4” and “00868582” in Form Locators 39-41. For claims submitted on the electronic claim 837I, the 8-digit number should be placed in Loop 2300 REF02 (REF01=P4) when a clinical trial claim includes condition code 30; ICD-9 code V70.71/ICD-10 code Z00.6 in either the primary or secondary positions; and, modifier Q0 and/or Q1, as appropriate (outpatient claims only).
For professional claims, the 8-digit clinical trial number preceded by “CT” (use “CT” only on paper claims) must be placed in Item No. 19 on paper claim Form CMS-1500 (e.g., CT00868582) or the electronic equivalent 837P in Loop 2300 REF02 (REF01=P4). Note: Do not use “CT” on the electronic claim, e.g., 00868582 when a clinical trial claim incudes: ICD-9 code of V70.7/ICD-10 code Z00.6 (in the primary or secondary positions); and, modifier Q0 and/or Q1, as appropriate (outpatient claims only).
Note: Medicare Part B clinical trial/registry/study claims with dates of service on and after January 1, 2014, not containing an 8 digit clinical trial number will be returned as unprocessable to the provider for inclusion of the trial number. Please see the following reference for copies of the MLM Matters Number: MM8401 Revised June 9, 2014 and CR Transmittal #: R2955CP released May 13, 2014 by clicking on the following link:
An example of a NaF-18 NOPR PET/CT whole body study is provided below that demonstrates the format and claim form location involved with reporting the clinical trial number. Also, the following link to the NOPR Educational Materials webpage provides links to complete examples of NaF-18 claim forms for both PI (initial treatment strategy) and PS (subsequent treatment strategy) in the hospital/facility and physician/IDTF provider settings.
https://www.cancerpetregistry.org/ed.htm
Download a complete copy of this Coding Tip: 8 Digit Registry Number Required on Clinical Trials and NOPR NaF Claims